Course 2 - The Periodic table and Periodicity
Hey guys, it's me again I am very happy to see you all Welcome to course two. Congrats🥳
We are going to tackle 8 sections, are you ready🤞.
The periodic law states that the properties of elements are a periodic function of their atomic numbers.
Atomic number determines the property an element possess. How so?
In the periodic table elements are arranged in increasing order of atomic number.
When elements are arranged in increasing order of their atomic numbers, their properties repeat at regular intervals (periodically). This is why elements with similar properties fall into the same group (vertical column) in the Periodic Table.
I will explain more as we go into other sections but what you should learn from this is that:
Both the physical and chemical properties of elements are determined by their atomic numbers.
Please😋Jot down what you learnt in your note book, this course 2 buckle up 🤬
The Modern Periodic Table
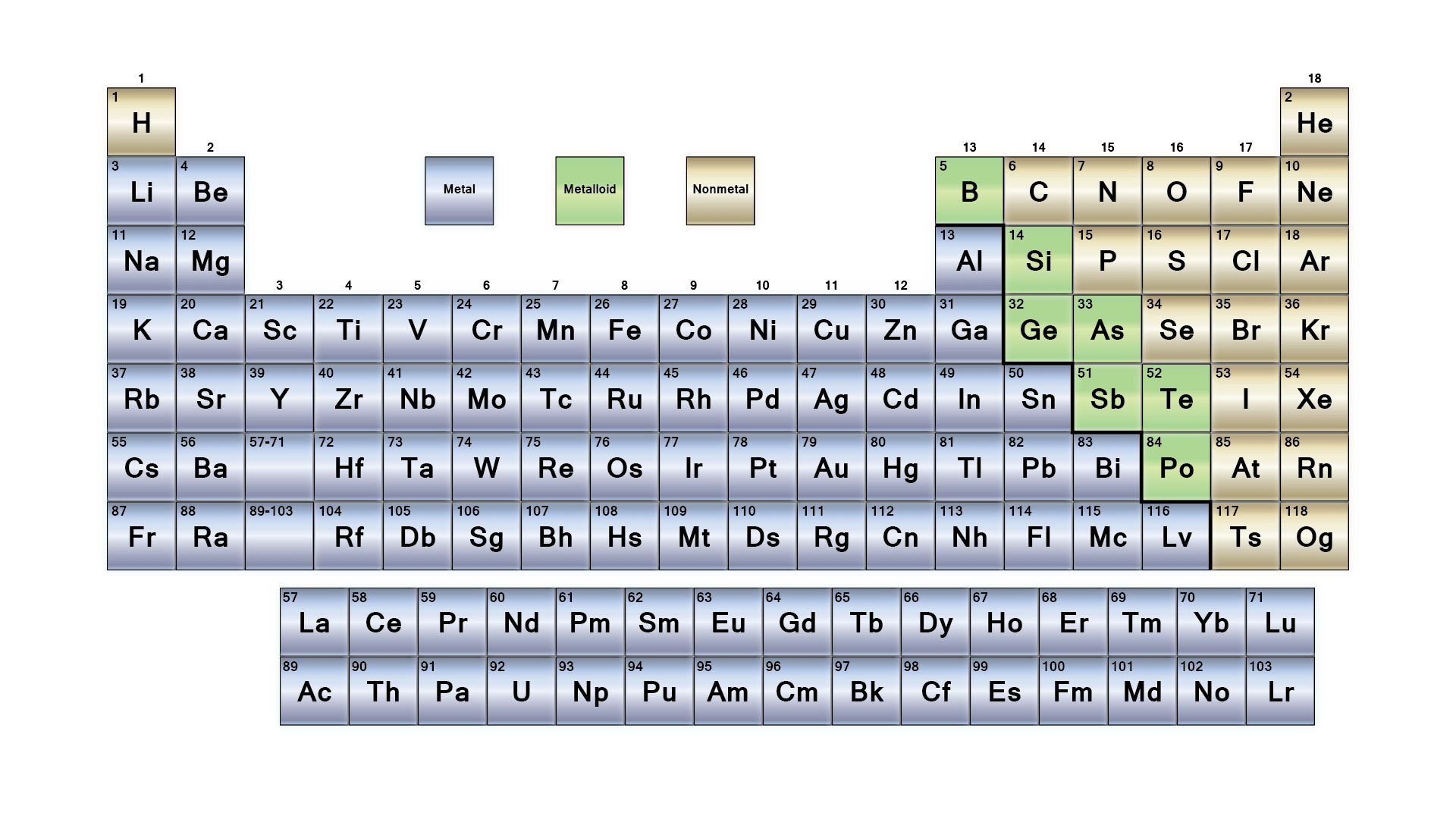
The image above is a representation of the period table, just type periodic table in google and you will see a more detailed one, alright.
There are a few things you need to know about the modern periodic table.
The periodic table was originally created by Dmitri Mendeleev, a Russian chemist, in 1869.
Henry Moseley, in 1913 discovered the concept of atomic number through his work with X-rays and restructured the periodic table based on this, which resolved inconsistencies in Mendeleev's version.
As you’ve learnt atomic number is everything to the periodic table.
The position of an element in the Periodic Table is determined by atomic number.
Let me break it down, the vertical columns in the table are called groups.
While the horizontal rows are called periods.
So, in other words, the periodic table is read in groups and periods.
In that video, i explained which parts are the groups and which are the columns.
What is the use of periodic table:
The periodic table organizes the elements and makes it easier for us to understand their relationships as they are in families. It also helps us to study trends of properties in among elements.
Thats all you should know about the periodic table for now.
Please😋Jot down what you learnt. Re read the points again, if its not clear.
Groups
Groups are the vertical columns in the periodic table. If you count them, there are 18 groups
But most times we exclude group 3 to 12, as they are transition elements and just say, that there are 8 groups, Group 1,2,3,4,5,6,7,0. Let’s learn about them all😙.
Group 1
- Group one elements are also called Alkali metals
- They all have 1 valence electron. (i.e. the number of electrons in their outermost shell)
- They are highly reactive because of that.
- They are soft metals.
- Lithium is used in batteries
- Na and k are used in our body
- They include: H, Li, Na, K, Rb, Cs, and Fr.
- Hydrogen is placed at the top of Group 1 because of its electronic configuration (1s1) However, it is often considered in a class of its own due to its versatility and behavior.
- First of all, it is not a metal, but a gas
- Secondly, group one elements lose their 1 valence electron, but hydrogen can gain or lose its electron.
Hydrogen is acknowledged as part of Group 1, but with a unique status.
Group 2
- Group two elements are also called Alkaline earth metals
- They have 2 valence electrons in their outermost shell
- They are also soft metals
- They are reactive but not as reactive as alkali metals
- They include: Be, Mg, Ca, Sr, Ba, and Ra.
Group one and two makes up the s-block. Meaning their valence electron is always in the s sub-shell (use ask now)
Try it out yourself.
Group transition elements (3 - 12)
- They are also known as transition metals.
- They are elements from group 3 to 12 and are found in the d-block.
- They have partially filled d-orbitals in at least one of their oxidation states.
- They exhibit transitions in their properties due to their partially filled d-block. For instance, many transition metals form colored compounds because of how their electrons behave.
Group 13, 14, 15, 16
If I am being honest, you gotta research that yourself.My main focus is for you to understand 1, 2, 3-12, 17 and 18. Thanks for understanding😎.
Group 17(Halogens)
- They are also known as Halogens.
- They have 7 valence electrons.
- They are non-metals.
- They are highly reactive.
- Reactivity decreases down the group, with fluorine being the most reactive halogen.
- They are great oxidizing agents.
- They include F, Cl, Br, I, At and Ts.
- F and Cl are gases, Br is a liquid, while I, At and Ts are solids.
Group 18 or Group 0(Noble gases)
- They are also called noble gases or rare gases.
- Noble gases are stable. They follow the octet rule (ask now) by having 8 valence electrons, except helium which has 2 valence electrons.
- Because they are stable, they do not react.
Please😋Jot down what you learnt. Re read the points again, if its not clear.
Periods
Periods are the horizontal rows in the table arranged in a way that:
- No of protons or atomic numbers increases.
- Atomic size decreases. (type “atomic size periodic table” In ask now)
There are seven periods.
Jot down what you learnt including what you learnt from ask now. Now it’s time for first test💀
Course 2 First Test
Read the questions carefully, then answer. Make me proud😁
Ionization energy
What is ionization energy?
Ionization energy is the energy required to remove the outermost electron from a neutral atom.
Trends in the periodic table
Across a period (left to right)
Ionization energy increases because the nuclear charge (number of protons) increases, pulling electrons closer and making them harder to remove.
Down a group
Ionization energy decreases because atoms get larger, and the outermost electrons are farther from the nucleus, so the nuclear pull weakens.
What is first ionization energy?
This is the energy required to remove the outermost, easy-to-take electron from an atom.
Difference between ionization energy and first ionization energy
Ionization energy is broad and can refer to any step of electron removal, whether it's the first, second, third, or beyond while First ionization energy specifically refers to the energy required to remove the very first (outermost) electron from a neutral atom.
Note that when we talk about ionization energy, we study it’s occurrence in the gaseous state because gases have weak bonds making it easier to study
Please study twice if you don’t get it, and jot down what you learnt😟.
Metals, Non-Metals, and Metalloids
Elements in the periodic table are broadly classified into three groups based on their physical and chemical properties:
Metals:
- Found on the left and center of the periodic table.
- Properties: Good conductors, malleable, ductile, shiny, and form basic oxides.
Examples of metals are: Gold (Au), Iron (Fe), Aluminum (Al), Copper (Cu)
Non-Metals:
- Found on the right side of the periodic table.
- Properties: Poor conductors, brittle, dull, and form acidic or neutral oxides.
- Examples of non-metals are: Oxygen (O), Sulfur (S).
Metalloids:
- Found along the staircase line between metals and non-metals.
- Properties: Exhibit properties of both metals and non-metals, often semiconductors.
- Example of metalloids are: Silicon (Si), Boron (B).
As usual write down what you have learnt from this section💀😋Lets keep levelling up.
Trends in the periodic table
I taught you right from the start that one of the beauty of the periodic table is that it is arranged in a way that gives us the ability to predict the property of elements. For instance, How we know that ionization energy decreases down the group and increases across the period.
That is an example of PERIODIC TRENDS
Periodic trends are the predictable pattern in the properties of elements as you move across a period or down a group
Key periodic trends
1. Atomic size or Atomic radius
Across a Period: Atomic size decreases because as the atomic number increases so does the nuclear charge(charge on the nucleus) increases, pulling electrons closer to the nucleus.
Down a Group: Atomic size increases (because new electron shells are added, increasing the size).
Example: Across a period
Lithium is bigger than Beryllium, Beryllium is bigger than boron...
Example: Down a group
Lithium is smaller than sodium, sodium is smaller than potassium...
Course 2 Second Test
Read the questions carefully, then answer. Make StudyHawkX happy😁
Key periodic trends cont'd
2. Ionization energy
Across a period: Ionization energy increases across a period (because the atomic size becomes smaller and tighter making it harder to pull electrons), therefore more ionization energy would be needed across a period.
Down a group: Ionization energy decreases because, (down a group more shells are added making the distance between electrons and the nucleus farther) therefore it would be easy to pull out electrons. So smaller ionization energy would be required down a groups
3. Electronegativity
This is the tendency of an atom to attract/gain electrons.
Across a Period: Increases (due to smaller atomic radius and stronger nuclear pull).
Down a Group: Decreases (due to increased atomic radius and weaker attraction for bonding electrons).
Example: Fluorine (F) is the most electronegative, while Cesium (Cs) is one of the least.
Note that
Electronegativity is the tendency of an atom to gain electrons to it.
Electropositivity is the tendency of atoms to lose electrons.
Most times metals are electropositive and most non-metals are electronegative.
For instance NaCL which one of them is losing electrons and which is attracting or gaining electrons. I leave that to you.
Jot down what you learnt its time to take the final test for course two🤓I REALLY WISH YOU THE BEST😊.
Chemistry's Course 2 Final Test
Read the questions carefully, then answer. Make me proud😁