Course 1 - The atomic structure
Matter
What exactly is matter?
Matter is anything that has mass and occupies space. For example, a pen, it has mass right? how do I know, because gravity pulls only what has mass so if you throw the pen, it will surely come down right? And the pen occupies space also, right? how do I know, when you put it on your table it covers a part of the table.
Think of matter as material. Anything in this world that has mass and occupies a given space is MATTER.
Examples:
Water, Air, rocks paper and so on.
Not everything is matter. For instance, Energy, forces vacuum therefore everything is not made up of matter, don’t get that twisted.
Mass is the amount of matter in a body.
Jot down what you learnt😁.
Elements
Elements are basically different types of matter. They are substances that cannot be split into simpler substances.
Think of them as substances in their pure form. They are made up of only one type of atom.
Let us use oxygen (You know that gas we breathe in).
Oxygen as an Element
Definition: Oxygen is an element because it is a pure substance that cannot be broken down into simpler substances by ordinary chemical means. If you divide oxygen, would you get chlorine inside it? No, it would still be oxygen
It is represented in the periodic table with the symbol O.
The first 20 elements according to the periodic table
| Elements | Symbols | Atomic number | RAM |
|---|---|---|---|
| Hydrogen | H | 1 | 1.008 |
| Helium | He | 2 | 4.0026 |
| Lithium | Li | 3 | 6.939 |
| Beryllium | Be | 4 | 9.0122 |
| Boron | B | 5 | 10.81 |
| Carbon | C | 6 | 12.011 |
| Nitrogen | N | 7 | 14.0067 |
| Oxygen | O | 8 | 15.9994 |
| Fluorine | F | 9 | 18.9984 |
| Neon | Ne | 10 | 20.183 |
| Sodium | Na | 11 | 22.9898 |
| Magnesium | Mg | 12 | 24.312 |
| Aluminum | Al | 13 | 26.9812 |
| Silicon | Si | 14 | 28.086 |
| Phosphorus | P | 15 | 30.9738 |
| Sulfur | S | 16 | 32.06 |
| Chlorine | Cl | 17 | 35.453 |
| Argon | Ar | 18 | 39.948 |
| Potassium | K | 19 | 39.102 |
| Calcium | Ca | 20 | 40.08 |
118 have been discovered by scientist, you can find them in the periodic table. (You will cover them in course two). Make sure to learn at least the first twenty elements its symbols and atomic numbers.
Some elements discovered long ago were named based on their Latin origins, which is why their symbols differ from their English names. For example:
These symbols preserve the historical roots of the elements while aligning them with modern naming conventions.
Jot down what you learnt🥲.
Atoms
What exactly is an Atom?
An atom is the smallest part of an element that still has the chemical properties of that element.
Take for example PAPER. Let us say that paper is an element and we cut that paper to the super tiniest piece, that super tiniest piece is an atom of paper. Atoms are what makes up matter (materials).
Because atoms still contain the chemical properties (use ask now) of that substance it can still take part in chemical reactions (use ask now).
Let’s go back to that paper, that very tiny piece of the paper can still burn, right?
Burning is a chemical reaction, do you get? Think about it.
Now there Is an issue, atoms cannot stand or survive alone🤔Wait, that’s actually a big issue so how do they survive then to make up our clothes, salt water and so on, well you will find out next section 😊.
Jot down what you learnt tho🥲.
Molecules
In the last section we found out that atoms can’t survive alone, so therefore they join together to form bonds, which we call MOLECULES.
Well from that we say that, Molecules are combinations of atoms. Simple as ABC. Molecules are combinations of atoms; it could be the same atoms like hydrogen and hydrogen combining to form H2 or Hydrogen combining with Oxygen to form H2O.
When the atoms are different, we no longer call them molecules but compounds (use ask now)
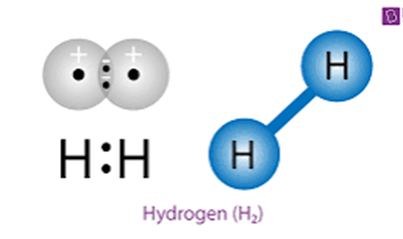
In the picture you can see two same H atoms combining to form H2. It is called hydrogen molecule
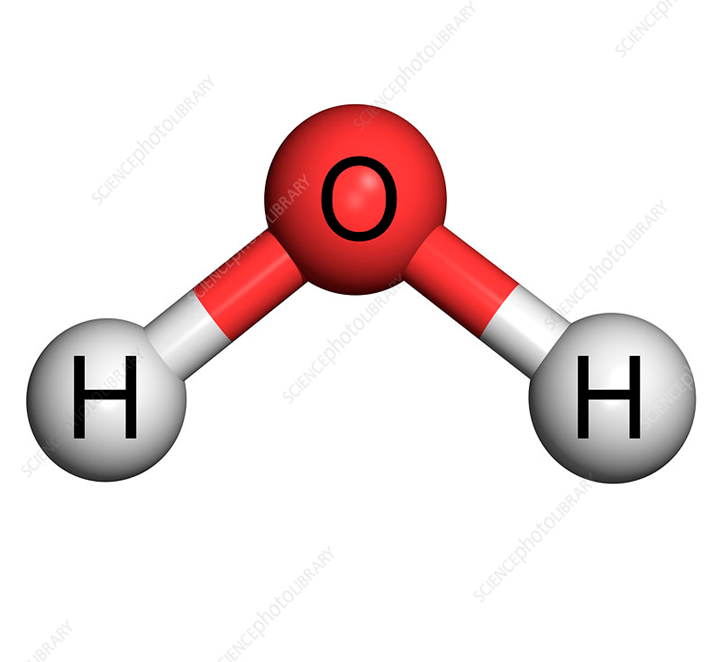
You can see clearly that two different atoms are combined hence we call it a compound.
Difference between a molecule and a compound
| MOLECULES | COMPOUNDS |
|---|---|
| Molecules are chemical combination of atoms of the same element. | Compounds are chemical combinations of atoms of different elements. |
| Not all molecules are compounds | All compounds are molecules |
Jot down what you learnt👌.
StudyHawkX First Test
Test your knowledge and see your score!
Subatomic particles
As tiny as atoms are, it can still be split into three more tinier particles we call SUBATOMIC PARTICLES. They are: protons, neutrons and electrons.
Protons are the positively charged particles in an atom.
Electrons are the negatively charged particles in an atom.
Neutrons are neutral i.e they have no charge
| Particle | Charge |
|---|---|
| Proton | +1 |
| Neutron | 0 |
| Electron | -1 |
Take a look at the image we can see the protons and neutrons joined together and the electrons orbiting them
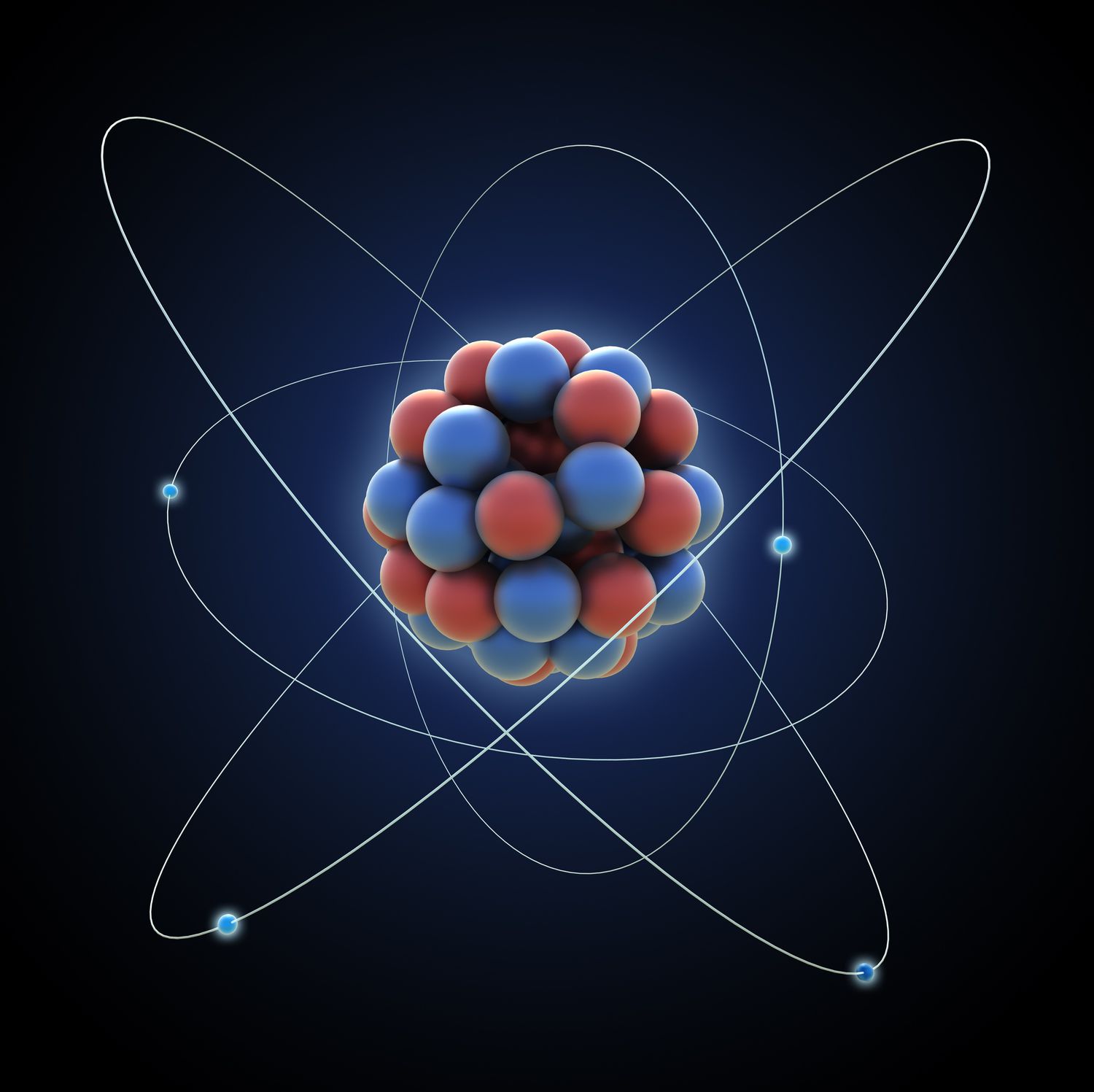
All you should know about the subatomic particles
Use ask now to learn everything you need to know about them.
Jot down what you learnt🥲.
Ions
What are ions?
Ions are atoms that have become electrically charged. Well, what the heck do I mean:
We all know that an atom consists of smaller particles.
Normally, an object is electrically neutral meaning the number of protons equals the number of electrons. Therefore, there is a balance.
Now an object is electrically charged when there becomes an imbalance, meaning the number of protons and electrons becomes different.
Let’s use helium atom, helium has 2 protons as well as 2 electrons. When we add it together, we get a neutral charge (+2 + -2 = 0) they cancel each other.
So, what do you think would happen when that helium atoms gains an electron, (+2 + -3 = -1) there would be more electrons making that atom negatively charged.
What if that helium atom loses an electron, (+2 + -1 =+1) there would be more protons making the atom positively charged.
So we no longer refer to such charged atom as just atom we call them IONS.
Types of ions
1. Cation: Positive ions.
2. Anion: Negative ions.
Note that an atom can only gain, or lose electrons because only electrons can move without much restriction.
Jot down what you learnt😎.
Electronic configuration
Welcome to one of the most important topics in chemistry😤, THE ELCTRONIC CONFIGURATION.
What is electronic configuration?
Electronic configuration means how electrons are arranged in an atom. Electrons are not just flying around like a bunch of crazy objects; they arrange themselves in what we call SHELLS OR ENERGY LEVELS. Think of it the planets orbit the sun they do so in an orderly way.
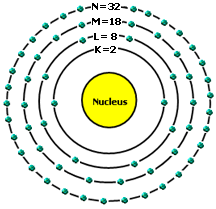
Take a look at the picture above: The nucleus is where the protons and neutrons are combined together. Then electrons orbit around the nucleus in KLMNO… shells.
The first shell or energy level is K and it can hold only 2 electrons.
The second shell is L and it holds only 8 electrons.
The third shell is M and it holds only 18 electrons.
The fourth shell is N and it holds only 32 electrons
It goes on like that.
Let’s take a look at the first three elements and how their electrons are arranged in an atom.
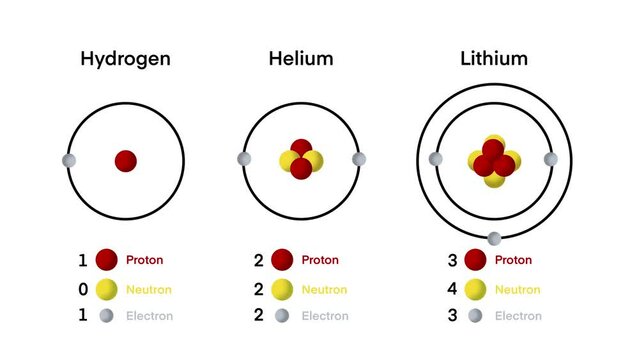
Do you see that, guess what shell they all are? H is in the K shell, He is in the K shell also, Li is in the L shell.
What are subshells?
Subshells are smaller parts inside a shell (like K, L, M). Each shell is split into subshells called s, p, d, and f, which are like sections where electrons are grouped. These subshells help us know exactly where electrons are in an atom and give a clearer picture of how they are arranged.
- s holds 2 electrons
- p holds 6 electrons
- d holds 10. electrons
- f holds 14 electrons
This is the pattern for writing the electronic configuration of atoms following the Aufbau principle (use ask now). Remember electronic configuration means how electrons are arranged.
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p …
This is the only thing you have to memorize during this course 🤥🤥.
Examples of electronic configurations:
1. H (hydrogen): The atomic number (use ask now) of H is 1 that tells us that there is also 1 electron. So:
1s1
2. Na (sodium): The atomic number of Na is 11 that tells us that there is also 11 electrons. So:
1s2 2s2 2p6 3s1
3. Neon (Ne): The atomic number of Nei is 10 that tells us that there is also 10 electrons. So:
1s2 2s2 2p6
4. B (boron): The atomic number of B is 1 that tells us that there is also 5 electrons. So:
1s2 2s2 2p1
5. Cl (chlorine): The atomic number of Cl is 17 that tells us that there is also 17 electrons. So:
1s2 2s2 2p6 3s2 3p5
Practice
Instructions: You are given the symbols of elements, write their electronic configurations in a notebook.
1. He
1s2
2. Be
1s2 2s2
3. O
1s2 2s2 2p4
4. Mg
1s2 2s2 2p6 3s2
5. Ca
1s2 2s2 2p6 3s2 3p 6 4s2
Explanation of Aufbau Principle and Exceptions
The Aufbau principle helps us predict how electrons fill atomic orbitals step by step, starting from the lowest energy levels. For most elements, this works perfectly. For example: - Calcium (Ca): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² (follows Aufbau)However, some elements like silver (Ag), copper (Cu), and chromium (Cr) are exceptions. This happens because:
1. Half-filled (d⁵) or fully filled (d¹⁰) orbitals are more stable.
2. To achieve this stability, an electron from the outer s-orbital shifts into the d-orbital.
For silver (Ag) (atomic number 47):
- Expected: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d⁹
- Actual: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s¹ 4d¹⁰
The shift makes the d¹⁰ orbital fully stable, even if it slightly breaks Aufbau's order. So, Aufbau is the rule, but we refine it with exceptions for stability.Jot down what you learnt pws🥱This was a long one.
Mass Number and Atomic Number
Remember atoms can be split into three subatomic particles, proton, neutron and electron.
Electrons contributes very little to the mass of the atom, it is the protons and the neutrons that carry the weight.
So when you see the mass number of X atom is 10, it means that the number of protons + the number of neutrons is equal to 10. Simple,
Let me break it down further (I’m here for you👌), Mass number is the addition of the atomic number and the neutron number in the nucleus of an atom.
Mathematically,
Mass number (A) = Atomic number (Z) Neutron number (N)
I.e. A = Z + N
let’s say X atom has 4 protons and 6 neutrons. The mass number of that X atom would be:
Z = 4 (protons)
N = 6 (neutrons)
A = 4 + 6
= 10//
Atomic number is the number of protons in an atom. Why is the number of protons called atomic number, it is because the number of protons determines what element that atom is.
For example: If you found an element and you discovered that it has only 1 proton meaning its atomic number is one, which element is that atom:
It’s Hydrogen, hydrogen has only one proton.
- Can you guess which element has 7 protons?
👉 It’s nitrogen (you’ll confirm this in the second test 😉).
Put this in your hearts
- . 126C 12(the superscript) represents the mass number while, 6 represents the atomic number of that carbon atom.
- Number of protons(atomic number) = Number of electrons
If protons are 5 then the electrons are also 5.
Make sure to Jot down what you learnt 🤓 LETS GOOO.
StudyHawkX Second Test
Test your knowledge and see your score!
Isotopy
Isotopy is a phenomenon or situation whereby atoms of the same element have the same number of protons but different numbers of neutrons.
Imagine an element X has two versions of its atom:
1. One with 5 protons and 5 electrons
2. The other with 5 protons and 6 electrons
They are both still X atoms but just different versions of it. Such situation is called isotopy.
So, from that what are isotopes?
Isotopes are atoms of the same element that has the same number of protons but different number of neutrons.
Meaning they have the same atomic number but, just different mass number because ATOMIC NUMBER IS UNCHANGEABLE, that’s how we determine the element it is.
This is how isotopes are represented.
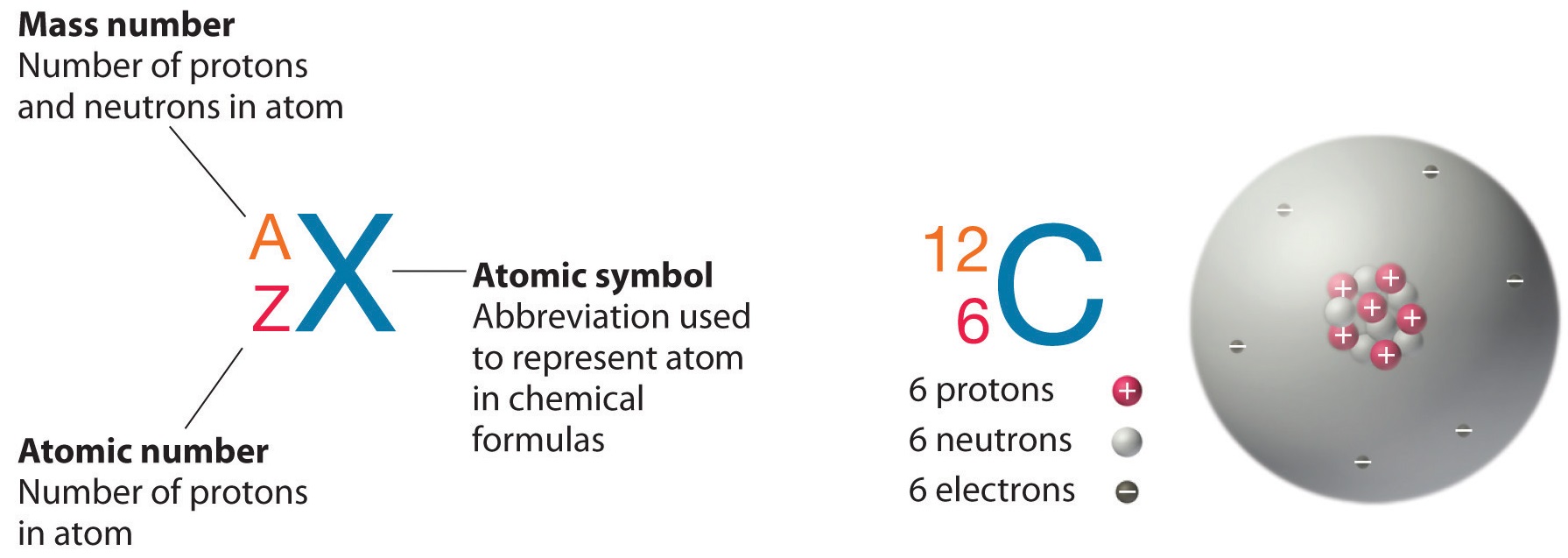
126C and 146C are isotopes of carbon.
Why are there Isotopes?
Isotopes exist because atoms of the same element can have different numbers of neutrons in their nuclei while still having the same number of protons. The number of neutrons can vary without changing the chemical properties of the element, but it affects the atom's mass and stability. This variation occurs naturally due to differences in how atomic nuclei form and bond during nuclear processes.
Jot down what you learnt, I beg you😭
Relative Atomic and Molecular mass
The mass of an atom is extremely small, and impractical to use directly in calculations.
For example, the mass of a hydrogen atom is 1.67×10-24 grams. You see that is very small and difficult to work with.
So scientists created the Relative Atomic mass system.
The relative atomic mass of an element is the average mass of one atom relative to 1/12th of carbon-12 atom.
The relative atomic mass (RAM) of hydrogen is approximately 1.008, which means the mass of a hydrogen atom is about 1.008 times the mass of 1/12 of a carbon-12 atom.
This system helps us easily compare the masses of different atoms relative to carbon-12, instead of using their extremely small absolute masses.
The mass spectrometer is used to measure relative atomic mass.
Relative molecular mass is the sum of the relative atomic mass of all the atoms in a molecule.
You calculate it by adding the RAM of all the atoms in a molecule.
E.g. H20(water)
RAM of H = 1
H2 = (1 × 2) = 2
RAM OF O =16
RMM = 2 + 16 = 18//
Therefore, the relative molecular mass of Water is 18.
Jot down important concepts you learned 😁.
Steps to Calculate RAM
1. Know the formula:
RAM = ∑ (Isotope Mass Number × Abundance) / ∑Abundance
2. Understand the terms:
- ∑ means to add or sum.
- Isotope Mass Number: The mass number of each isotope.
- Abundance: How much of each isotope exists, usually given in percentages or fractions.
3. So basically, when you want to know the ram of the isotopes of an element you multiply each isotope's mass number by its abundance in nature. Then add them together.
Finally, divide by the total abundance (100% or 1, depending on the units).
Example: Chlorine
- 35Cl: Mass = 35, Abundance = 75%
- 37.Cl: Mass = 37, Abundance = 25%
Step-by-step Calculation:
(35×75) + (37×25)
= 2625 + 925
=3550
75 + 25 =100%
3550/100 = 35.5
Work to do
1. What is the RAM of 126X and 136X. If its abundance in nature is 60% and 40%.
Solution
Mass Number of isotope 1 = 12, Abundance in nature = 60%
Mass Number of isotope 2 = 13, Abundance in nature = 40%
RAM = ∑ (Isotope Mass Number × Abundance) / ∑Abundance
12 × 60 + 13 × 40 = 720 + 520
= 1,240
60 + 40 = 100
1,240/100
= 12.4
Therefore, the relative atomic mass of X atoms is 12.4//
If you got it, congrats🥳. Keep levelling up
2. What is the RAM of 2010X and 2210X. If its abundance in nature is in the ratio 3:1.
Solution
If you are given ratios instead of percentage, first convert it to percentage
1. Total ratio = 3 + 1 = 4
2. 100%/4 = 25%
3. 25% 3 = 75% and 25% 1 = 25%
4. Therefore, their abundance in nature is 75% and 25%
5. Now we can calculate,
RAM = ∑ (Isotope Mass Number × Abundance) / ∑Abundance
20 × 75 + 22 × 25 = 1500 + 550
= 2,050
60 + 40 = 100
2,050/100
= 20.5
Therefore, the relative atomic mass of X atoms is 20.5//
If you got it, congrats🥳. You are really on the right track then.
3. How many neutrons does X element have if 22/10X?
Solution
A = 22
Z = 10
N =?
Mass number = Atomic number + Neutron number
A = Z + N
Since we are looking for the value of N,
N = A – Z
N = 22 – 10
= 12
Therefore the number of neutrons present are 12//
Jot down what you have learnt in your StudyHawkX notebook🤞
Mole
A mole is a unit in chemistry used to measure the amount of substance.
Just like Dozen which means 12, mole means 6.022 10^23. We call that value Avogadro’s constant (use ask now).
1 mole of water means 6.022 10^23 water molecules.
Moles is used because it is hard to count small atoms so we use moles to count large amount of it instead.
Jot down what you have learnt in your StudyHawkX notebook🤞.
Molar Mass
Molar mass is the mass of one mole of a substance.
It is measured in g/mol.
g/mol corresponds to atomic mass units amu (use ask now), but g/mol is in greater magnitude (plenty atoms) while amu is just one atom.
The Molar mass of carbon is 12g/mol same as the RAM of carbon which is also 12 amu.
They are both 12 but of different magnitude.
RMM OF H2O = 18 amu
Molar mass of H20 = 18g/mol.
Jot down what you have learnt in your StudyHawkX notebook🤞.
Chemistry's Course 1 Final Test
Read the questions carefully, then answer. Make me proud😁